면역항암치료(Cancer immunotherapy)
최근 의과학 기술의 발달로 암치료의 새로운 패러다임으로 DNA 기반 암백신(DNA-based cancer vaccine) 전달 체계 등을 이용한 면역항암치료(cancer immunotherapy)가 주목을 받고 있다. 면역항암치료는 우리 몸의 면역세포를 활성화시켜서 암을 치료하겠다는 개념으로 면역체계 구성요소에 관여하여 암 환자 치료를 위한 것으로 개념이 소개된 지는 19세기 후반 William B Coley가 Coley 독소로 알려진 박테리아 혼합물 투여 후 종양 감소를 처음 관찰한 이후 백년이 넘었으나, 실제로 이러한 개념들이 임상에 입증되는데는 상당히 오랜 시간이 걸리게 되어 면역항암제가 임상에 제대로 도입된 시기는 2011년부터이다. 면역항암치료는 미국 전 대통령 지미 카터(Jimmy Carter)가 면역항암치료로 암을 완치했다는 소식과, 2018년 면역항암제 치료효과와 관련된 노벨상 소식 등으로 기대가 높아지고 있다.
면역항암요법은 인체의 면역체계를 활성화시켜 자가면역력을 높여서 면역세포가 암세포를 공격하도록 하는 치료법으로, 기존의 방사선요법, 세포독성 치료제, 표적항암제와 작용 메커니즘부터가 다르다. 기존의 항암제는 암세포의 DNA 또는 발현 단백질을 타겟으로 하여 (즉, 암세포가 발현하는 특정 돌연변이 유전자 혹은 변형된 단백질) 공격하는 반면에, 면역항암제는 면역세포의 기능에 집중하여 면역세포의 ‘암세포를 공격하는 잠재력’을 깨우는 것이다. 또 다른 차이점은 기존의 항암제는 암세포 자체를 타겟으로 하지만 면역항암제는 암세포뿐만이 아니라 암 주변에 존재하는 세포, 종양미세환경(tumor microenvironment)를 조절한다.
이러한 작용메커니즘의 차이는 치료효과에도 차이를 보인다. 1차 항암치료제인 세포독성항암제는 2~3개월의 치료효과를, 2차 항암치료제인 표적항암제는 치료 지속기간이 10-12개월을 나타낸 반면에 3차 항암치료제인 면역항암제의 경우 그 효과가 반응기간을 가늠할 수 없을 정도로 장기적으로 지속된다. 이렇게 장기적으로 지속가능한 효능을 갖을 수 있는 이유는 바로 면역세포의 기억 능력 때문이다. 면역항암제에 의해 암세포 공격 기능이 향상된 면역세포는 암세포가 그 기능 및 성질을 완전히 바꾸지 않는 한 처음 공격했던 암세포를 기억하고 지속적으로 공격한다. 또다른 이유는 면역항암제는 내성에 대한 우려가 적다는 점이다. 내성이 전혀 없다고는 말할 수 없지만 투여 후 1년이내에 내성이 나타난다고 알려져 있는 대표적인 표적치료제인 EGFR 억제제와 비교하였을 때 면역치료제는 투여 후 내성이 생기기까지의 기간이 길다. 또한 부작용이 적다는 점을 들 수 있다. 표적항암제 중에서 정상세포에 대한 독성이 가장 적다고 알려져 있는 EGFR 억제제의 경우 투여환자의 20~30%가 피부트러블, 간지러움 등의 부작용을 경험한다. 물론 면역항암제 투여환자의 20%가 피로감을 경험하며, 10~20%가 피부발진을 보이지만 간지러움의 강도 및 빈도가 표적항암제보다 낮다고 알려져 있다. 이러한 점을 통하여 면역항암제는 생존기간을 대폭 연장시킬 뿐만 아니라 환자의 삶의 질을 높이는 효과를 갖는다고 할 수 있다.
일부 면역항암요법은 암세포에 의해 발현하는 단백질에 결합하여 그 기능을 억제하는 항체로 구성된다. 다른 면역항암요법에는 크게 백신 및 T 세포 주입이 포함된다. 인체 내부의 면역조절은 수지상세포, T 세포, B 세포, NK 세포로 대표되는 면역세포의 작용에 의해 이루어진다. 외부 물질이 체내로 들어오거나 내부물질이 변화되었을 때 체내의 면역시스템을 감시하는 역할을 하는 수지상세포(dendritic cell 또는 antigen presenting cell)가 가장 먼저 이러한 물질을 침입자로 인식한 후 조력자 T 세포 (helper T cell) 또는 세포독성 T 세포 (cytotoxic T cell)를 끌어당긴다.
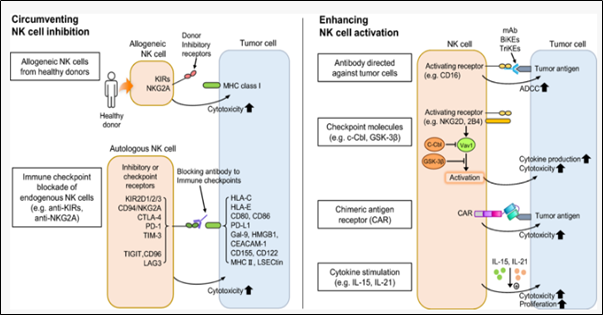
그림 1. 암 세포에 대한 NK 세포 반응성을 향상시키는 전략 종양 미세 환경 (왼쪽 패널)에서 NK 세포의 억제를 회피하거나 NK 세포의 활성화 및 종양 세포 (오른쪽 패널)에 대한 특이성을 강화하는 두 가지 전략에 의해 종양 세포에 대한 NK 세포 반응성을 복원하거나 향상시킬 수 있다(Archives of Pharmacal Research, 2019 42(7), 591)
위의 그림에서와 같이 암세포에 대한 NK 세포 반응성을 향상시키는 전력과 면역 체계 회피 반응을 무력화하기 위해서 개발된 신약들은 크게 3군데를 타겟으로 하게 되는데, 아래 그림 2의 CTLA-4, PD-1, PD-L1이 해당된다. 신약들은 해당 타겟에 대한 specific antibody (특이 항체)로, 타겟과 결합하여 신호 전달을 차단하고 자아로 인식하여 면역회피를 하지 못하게 함으로써, 암세포가 정상적으로 T cell에 의해 제거될 수 있도록 하는 전략을 쓴다. 즉 이러한 신약은 면역체계가 정상일 경우에만 효과적으로 작동할 수 있다.
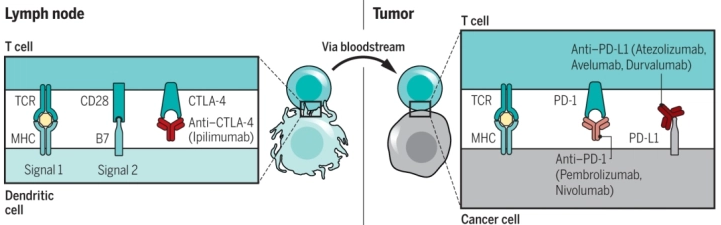
그림 2. Blockade of CTLA-4 and PD-1 to induce antitumor response (left). CTLA-4 is a negative regulation of costimulation that is required for initial activation of an antitumor T cell in a lymph node upon recognition of its specific tumor antigen, which is presented by an antigen-presenting cell. The activation of CTLA-4 can be blocked with anti-CTLA-4 antibodies (right).
인체는 외부의 바이러스, 독소 등으로 부터 위해뿐만이 아니라 내부의 정상세포의 암세포 변이 등의 해로운 변화로부터 스스로를 보호하는 방어체계를 갖추고 있다. 세포독성 T 세포는 직접적으로 침입자를 제거하는 역할을 하고, 조력자 T 세포는 B 세포를 유도하여 B 세포가 항원에 대항하는 항체를 분비하여 침입자항원을 제거하고, 침입자항원에 대한 기억을 저장하였다가 차후에 동일한 항원이 다시 침입하면 즉각적으로 방어를 할 수 있도록 하는 기능을 갖는다. NK 세포(natural killer cell)는 정상세포에 발현되어 있는 펩타이드의 일종인 MHC1을 인지하는 기능을 갖는데, MHC1이 발현되어 있지 않은 세포를 비정상 세포로 판단하여 공격한다. 이러한 면역세포의 기능을 조절하여 암세포를 공격하거나 암세포의 성장을 억제하도록 하는 것이 면역치료법의 기본바탕이 되는 개념이다.
면역항암제는 크게 면역관문억제제(immune checkpoint inhibitor)와 면역세포치료제(immune cell therapy), 치료용 항체(therapeutic antibody), 항암백신(anticancer vaccine)으로 분류할 수 있다. 면역관문억제제는 T 세포 억제에 관여하는 면역체크포인트 단백질(immune checkpoint protein)의 활성을 차단하여 T 세포를 활성화시켜 암세포를 공격하는 약제로서 대표적으로 CTLA-4, PD-1, PDL1을 인식하는 항체를 사용한다. 면역세포치료제는 환자 체내의 T 세포를 채취하여 강화 및 변형시켜 다시 체내에 주입하여 암세포에 대한 세포성 면역을 강화시키는 약제로 NK 세포치료제, T 세포치료제, CAR-T 세포치료제 등이 있다. 또한 치료용 항체는 항체-약물결합체가 암세포에 결합하면 약물이 유리되어 암세포를 공격하는 약제로서 항체-약물접합체(antibody-drug conjugate, ADC)를 대표적인 예로 들 수 있다. 항체-약물접합체는 치료용 항체에 약물을 접합시켜 암세포들을 파괴하도록 디자인된다. 즉 항체-약물 접합체의 항체가 암세포의 표면에 발현되는 표적분자에 반응하면서 암세포가 약물인 독성물질에 노출되어 사멸되도록 하는 치료법이다. 마지막으로 항암백신은 암세포가 갖고 있는 종양특이적 항원 또는 체내 전반적인 면역반응을 향상시킬 수 있는 단백질/펩타이드 분자를 암환자에게 투여하여 면역체계를 활성화시킴으로써 체내 면역기능이 활발하게 만들어 암세포를 공격하도록 하는 면역치료법이다. 항암백신은 암세포의 특정 항원을 대상으로 하여 암세포 특이적 면역반응을 유도하는 특이적 항암치료백신(펩타이드 백신, DNA 백신, 인유두종바이러스 백신 등)과 체내 전반적인 면역반응을 향상시켜 암을 치료하는 비특이적 항암치료백신(B7, TNF, 사이토카인 등)으로 나눌 수 있다.
위와 같은 면역 항암제로 개발된 약물의 종류를 요약하면 아래와 같다.
PD-1 inhibitors
-Pembrolizumab (Keytruda)
-Nivolumab (Opdivo)
-Cemiplimab (Libtayo)
이러한 약물은 피부 흑색종(melanoma of the skin), 비소세포폐암(non-small cell lung cancer), 신장암(kidney cancer), 방광암(bladder cancer), 두경부암(head and neck cancers) 및 호지킨 림프종(Hodgkin lymphoma)을 비롯한 여러 유형의 암 치료에 도움이 되는 것으로 나타났다. 이러한 약물들은 또한 다른 많은 종류의 암치료를 위해 연구되고 있다.
PD-L1 inhibitors
-Atezolizumab (Tecentriq)
-Avelumab (Bavencio)
-Durvalumab (Imfinzi)
이러한 약물은 방광암(bladder cancer), 비소세포폐암(non-small cell lung cancer), 메르켈 세포 피부암 (Merkel cell carcinoma)을 비롯한 여러 유형의 암 치료에 도움이 되는 것으로 나타났다. 이러한 약물들은 또한 다른 많은 종류의 암치료를 위해 연구되고 있다.
Drugs that target CTLA-4 blocker
-Ipilimumab (Yervoy)
아래 표 1에서 나타나는 바와 같이 면역함암제로 개발된 약물의 반응율은 질병에 따라 다르데 나타나는데, Hodgkin 종양, melanoma, MSI-high 종양의 면역 항암제 반응률이 좋은 편이고, 기타 두경부암, 소화기계 암들의 반응률은 20% 내외로 반응하고 있음을 보고하고 있다.
표 1. 암종별 면역 항암제 반응 정도
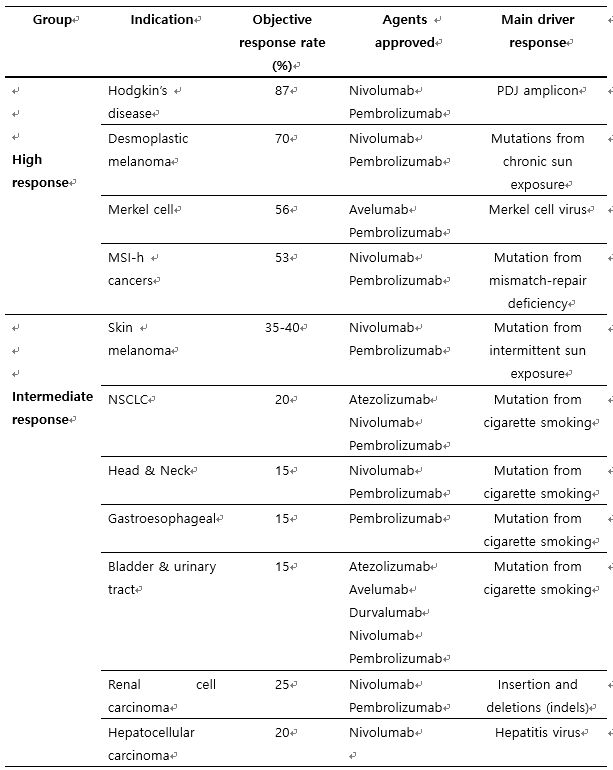
표2. 항암면역치료 유형(Categories of cancer immunotherapies)

면역 요법에는 다양한 방식으로 진행되는 치료법이 포함된다. 일부는 매우 일반적인 방법으로 신체의 면역 체계를 향상시키는 방법이고, 다른 방법은 암세포를 특이적으로 공격하기 위해 면역체계를 훈련시키는 것을 도와준다. 지난 수십 년 동안 항암면역요법은 암 치료에 중요한 부분이되어왔으며, 최근 새로운 유형의 면역 치료법이 지속적으로 개발 연구되고 있으며 미래의 암 치료법에 커다란 영향을 미칠 것이다.
KCT 임상연구자료 더보기 http://kct.medric.or.kr

References
Alvaro J. Alencar & Craig H. Moskowitz (2019). Immune-checkpoint inhibition as first-line therapy for Hodgkin lymphoma, Nature Reviews Clinical Oncology
Ribas, Antoni, and Jedd D. Wolchok. “Cancer immunotherapy using checkpoint blockade.” Science 359.6382 (2018): 1350-1355.
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480, 480–489 (2011)
Palucka, K., Banchereau, J. & Mellman, I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 33, 464–478 (2010).
Bayas, J. M., Costas, L. & Munoz, A. Cervical cancer vaccination indications, efficacy, and side effects. Gynecol. Oncol. 110, S11–S14 (2008).
Restifo, N. P., Dudley, M. E. & Rosenberg, S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Brentjens, R. J. et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828 (2011)
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012)
Dammeijer, F., Lau, S. P., van Eijck, C. H. J., van der Burg, S. H. & Aerts, J. Rationally combining immunotherapies to improve efficacy of immune checkpoint blockade in solid tumors. Cytokine Growth Factor Rev. 36, 5–15 (2017).
What is cancer immunotherapy? American cancer society: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/what-is-immunotherapy.html
Immune checkpoint inhibitors to treat cancer:
https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/immune-checkpoint-inhibitors.html
Recent Updates in Cancer Immunotherapy (2015). Korean ournal of Otorhinolaryngology-Head and Neck Surgery, 58(7): 449-455. doi: https://doi.org/10.3342/kjorl-hns.2015.58.7.449
Cancer immunotherapy: https://www.nature.com/subjects/cancer-immunotherapy