필라델피아 염색체 양성 백혈병(Philadelphia chromosome-positive leukemias)을 가진 환자의 신장기능에 대한 보스티닙효과
Effects of Bosutinib Treatment on Renal Function in Patients With Philadelphia Chromosome-Positive Leukemias
|
연구질문
|
필라델피아 염색체 양성 백혈병[Philadelphia chromosome-positive, (Ph+) leukemias] 환자에게 보수티닙(bosutinib) 투여는 신장기능에 효과가 있습니까?
|
|
답변
|
장기간의 보스티닙 치료는 이전의 TKI 치료에 저항성과 내성을 가진 만성기(CP)의 만성골수성 백혈병(Chronic Myeloid Leukemia, CML), 혹은 진행성 환자에서 신장기능의 가역적 감소와 관련이 있는 것으로 나타났다..
|
|
서지정보
|
Effects of Bosutinib Treatment on Renal Function in Patients With Philadelphia Chromosome-Positive Leukemias. Clinical Lymphoma, Myeloma & Leukemia, 2017-10-01, 17(10), 684-695. https://doi.org/10.1016/j.clml.2017.06.001
|
|
연구목적
|
필라델피아 염색체 양성 백혈병(Ph+) 환자의 신장기능에 대한 보수티닙(bosutinib) 혹은 이마티닙(imatinib.)치료에 대한 신장기능을 평가하기 위함
|
|
연구설계
|
Retrospective analysis of data from 2 open-label, multinational study
|
|
연구대상
|
First-line bosutinib (n = 248) or imatinib (n=251; phase III trial) => (N=499)
Second-line or later bosutinib (phase I/II trial; N= 570).
|
|
시험군 중재
|
First-line bosutinib (n = 248) or, second-line or later bosutinib (n = 570).
|
|
대조군 중재
|
Imatinib (n = 251)
|
|
평가지표
|
Incidence of renal adverse events (AEs)
Estimated Gomerular filtration rate (eGFR) rate
Serum creatinine
|
|
주요결과
|
-Incidence of renal adverse events (AEs): 두 번째 또는 그 이후의 보스티닙을 투여받은 (73/570명), 13%환자에서 나타났고, 첫 번째 보스티닙과 이마티닙을 투여받은 [22/248, (9 %) 16/251, (6 %)] 환자에서 보고되었다.
-Bosutinib을 투여받은 환자의 eGFR은 2 차 또는 그 이후의 bosutinib (139/570, 24 %)로 grade 3b eGFR (신장 질환에서의 식이요법 변경에 따라 45 mL / min / 1.73 m2 미만) )과 1 차 보스티닙 (26/248, 10 %) 및 이마티닙 (25/251, 10 %)치료와 비교했을 때; Grade_3b eGFR은 세컨라인 혹은 그 이후의 보스티닙 투여 시에 가장 짧게 나타났다.
|
|
근거수준
|
Moderate
|
|
작성자
|
의과학연구정보센터(MedRIC)
Copyright © 2015. Medical Research Information Center (MedRIC) Editors
|
표1) Characteristics and Management of Renal Adverse Events


그림 1) Median Change From Baseline in eGFR (A) and Serum Creatinine (B) Over Time in Both Studies. Baseline Was Defined as the Last Nonmissing Value Before the First Dose of Test Drug. Analyses for Each Time Interval Were Done Using Data With Nonmissing Baseline and Postbaseline Values. Per Protocol, Assessments at Months 27, 33, and 39 Were Not Done in the Phase I/II Study. 1 Month [ 4 Weeks. Nonparametric 95% Confidence Intervals Provided for the Median
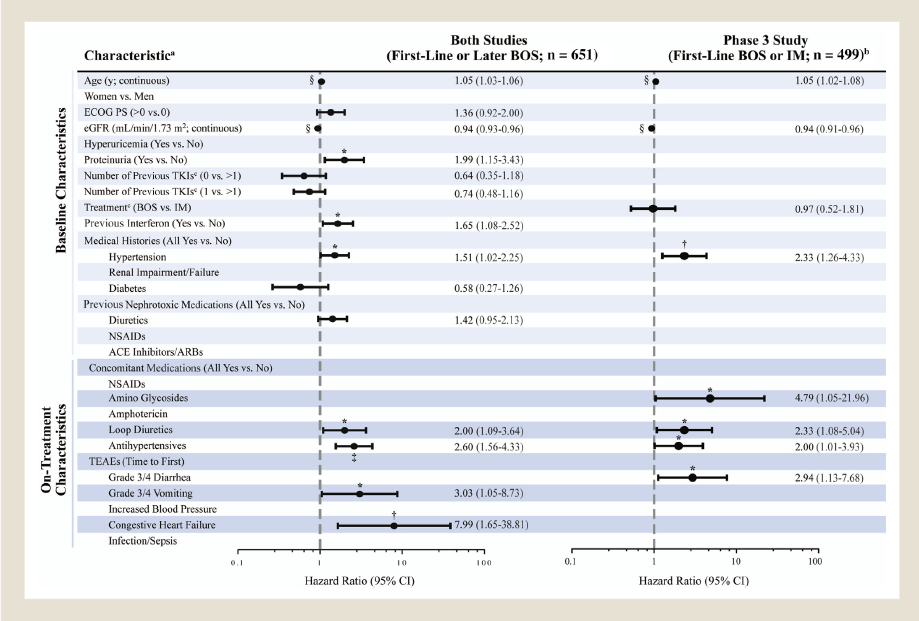
그림 2) Baseline and On-Treatment Time-Dependent Predictors of Time to First Grade ‡ 3b eGFR in CP CML Patients. Hazard Ratios < 1 Indicate Better Outcome for Group 1 Versus Group 2 (ie, Reference) or for a 1-Unit Increase for the Continuous Variables; 95% Confidence Intervals From the Cox Model Provided. Terms Used to Define Medical Histories Are Included in the Footnotes of Table 1. P Values Were Not Adjusted for Multiple Comparisons (*P < .05; yP £ .01; zP £ .001; xP £ .0001). eGFR Was Calculated on the Basis of the Modification in Diet in Renal Disease Method. Grading Is on the Basis of Kidney Disease Improving Global Outcomes Criteria Where Grade ‡ 3b is < 45 mL/min/1.73 m2 and Grade £ 3a Is ‡ 45 mL/min/1.73 m2. Patients Without Grade ‡ 3b Were Censored at the Last on-Treatment Laboratory Assessment. aForward Selection Was Used to Select Covariates for Inclusion in the Final Model (P[.20; Treatment Forced Into the Model for the Phase III Study); Maximum Likelihood Estimates, P Values, and Hazard Ratios Are Shown for Covariates Included in the Final Model. bData From the BOS and IM Treatment Arms Were Combined in the Model for the Phase III Study. cNumber of Previous TKIs (0 vs. > 1 and 1 vs. > 1) Was Included Only in the Model for the Phase I/II Study; Treatment (BOS vs. IM) Was Included Only in the Model for the Phase III Study. These Covariates Were Included in the Respective Models Regardless of Their Statistical
significance
Reference:
Effects of Bosutinib Treatment on Renal Function in Patients With Philadelphia Chromosome-Positive Leukemias. Clinical Lymphoma, Myeloma & Leukemia, 2017-10-01, 17(10), 684-695.
Bosutinib treatment for Philadelphia chromosome-positive leukemias.Future Oncol. 2014 Feb;10(2):179-85. Doi: 10.2217/fon.13.268.